Why is Glutamic Acid Residue Important in Protein Structure?
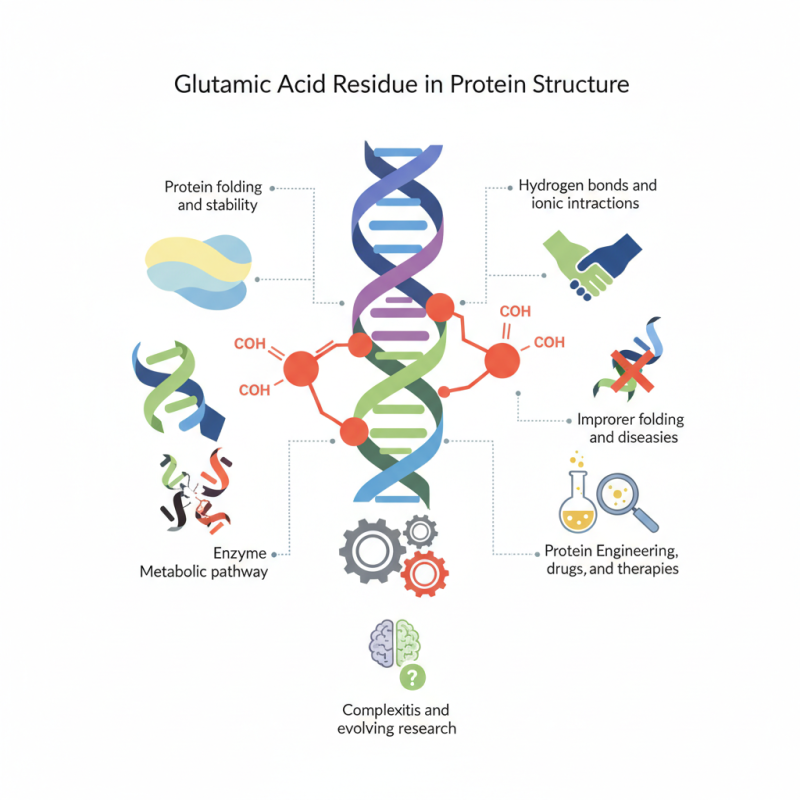
glutamic acid residue plays a vital role in protein structure. It is one of the 20 standard amino acids. This residue is unique due to its side chain carboxyl group. This property can influence protein folding and stability. Proteins often require a precise arrangement of residues. Glutamic acid can form hydrogen bonds and ionic interactions.
These interactions often dictate how proteins function. The presence of glutamic acid can alter protein conformations. It can also affect enzyme activity, influencing metabolic pathways. Moreover, improper folding is a common issue in biological systems. This can lead to diseases. Understanding the significance of glutamic acid residue enhances our grasp of these processes.
Studying this residue provides insights into protein engineering. Such knowledge is crucial for developing drugs and therapies. However, complexities arise in predicting protein behavior. Variability in environments can lead to different outcomes. Hence, research in this area is still evolving. Glutamic acid residue, while simple, is key to understanding life at the molecular level.
Role of Glutamic Acid Residues in Protein Folding Dynamics
Glutamic acid residues play a vital role in the protein folding dynamics. These residues are often found on the surfaces of proteins, where they interact with their environment. Their acidic nature contributes to the formation of hydrogen bonds. This interaction is crucial for stabilizing protein structures.
In folding, glutamic acid acts as a hinge. It helps proteins shift between different conformations. This ability to change shape is essential. Sometimes, the folding process can be inefficient, leading to misfolded proteins. When that happens, cellular functions may be disrupted.
Tips: Focus on the environment influencing protein behavior. Understanding how pH affects protein shape can provide insights. Additionally, consider how different temperatures can impact folding. Small changes may lead to significant effects on protein stability. Keeping these factors in mind allows for a more nuanced approach to studying proteins.
Impact of Glutamic Acid on Protein Stability and Function
Glutamic acid plays a crucial role in protein stability and function. As a negatively charged amino acid, it contributes to the overall structure of proteins. This charge can influence the folding of proteins. A well-folded protein is essential for its activity. Misfolded proteins can lead to diseases.
In many enzymes, glutamic acid is key to catalytic activity. Its side chain can form hydrogen bonds and ionic interactions. This interaction stabilizes the active site. Without glutamic acid, enzyme efficiency may suffer. It’s interesting to note that not all proteins utilize glutamic acid in the same way. Some show a clear dependence, while others do not.
The presence of glutamic acid also affects protein interactions. It can participate in forming salt bridges with positively charged residues. These connections can enhance the protein's structural integrity. However, the requirement for glutamic acid isn't universal. Some proteins function well without it. This variance poses questions about protein evolution and adaptation. As much as we admire the complexity of proteins, there remains a lot we still do not understand.
Interactions of Glutamic Acid with Other Amino Acids
Glutamic acid, with its carboxyl group, plays a crucial role in protein interactions. It often forms hydrogen bonds with other amino acids. This interaction stabilizes protein structure, creating functional domains. For instance, when glutamic acid interacts with lysine, it can form salt bridges. These connections are essential for maintaining protein shape.
The unique side chain of glutamic acid can also participate in enzymatic reactions. It helps in binding substrates. However, its negative charge can sometimes lead to repulsive forces. This can destabilize certain structures within proteins. Understanding these contradictions is vital for comprehending protein behavior.
Consider how these interactions influence enzyme efficiency. The subtle balance of attraction and repulsion affects catalytic activity. While glutamic acid is a tool for stability, it can also introduce vulnerability. This complexity mirrors the broader intricacies of protein dynamics.
Biological Significance of Glutamic Acid in Enzyme Catalysis
Glutamic acid is a crucial amino acid in protein structure. Its role in enzyme catalysis cannot be overstated. This residue contributes to the active site of many enzymes. In particular, glutamic acid can serve as both an acid and a base. This dual functionality is vital for the catalytic process. Studies show that about 30% of all enzymes utilize glutamic acid residues.
The importance of glutamic acid extends to its involvement in stabilizing transition states. These states are necessary for chemical reactions to proceed. The energy barrier is lowered, enhancing reaction rates. Research indicates that this amino acid significantly impacts enzyme efficiency. For instance, enzymes with glutamic acid residues can achieve turnover rates up to 10,000 molecules per second. Yet, not all proteins utilize this residue effectively. Some proteins lack the optimal conditions to fully harness its potential.
When considering enzyme design, the placement of glutamic acid is critical. Misplacing this residue can lead to ineffective catalysis. It raises questions about evolutionary pressures on protein structure. Understanding these dynamics remains a challenge. There is still much to learn about the precise roles of glutamic acid in various biochemical pathways. This complexity highlights the need for ongoing research into protein functions.
Implications of Glutamic Acid Deficiency in Health and Disease
Glutamic acid, an amino acid, plays a crucial role in protein structure. It is vital for the proper functioning of many proteins. However, a deficiency in glutamic acid can lead to serious health issues. Low levels of this amino acid might impact neurotransmitter function. This can result in mood disorders and cognitive decline.
Our diets should ideally include sufficient glutamic acid. It is found in many protein-rich foods like meat, fish, and dairy. Vegetarians can get it from legumes, nuts, and seeds. If you feel fatigued or mentally foggy, consider evaluating your diet.
Tips: Incorporate more protein sources into your meals. Experiment with new recipes that include legumes or fish. Regularly check your mental health and be aware of any changes. Balance is key, but it might not always be easy to achieve. Recognizing gaps in your nutrition is a great step toward better health.

